Session VI - Reconstruction
Bone Strain Changes Associated with Intraarticular Fractures of the Proximal and Distal Tibia
Todd O. McKinley, MD; Anthony N. Brown, MD; Peter W. Callendar, MD; Brian K. Bay, PhD, University of California at Davis, Sacramento, CA
Purpose: The etiology of post-traumatic arthritis remains unknown but it clearly has a pathomechanical component. Pilon fractures (43 B,C) often develop early, aggressive post-traumatic arthritis, in contrast to tibial plateau fractures (41 B,C), which degenerate slowly. Cartilage, calcified cartilage, the subchondral plate, and trabecular bone form a composite periarticular structure that is injured by intraarticular fractures, frequently leaving defects in the cartilage and subchondral bone. Understanding how cartilage and subchondral bone defects affect the mechanics of the periarticular structural complex would give new insight into the pathomechanics of post-traumatic arthritis. The purpose of this study was to determine how intraarticular fractures of the proximal and distal tibia affect load transmission through the periarticular composite structure by direct measurement of changes in trabecular bone strain that result from cartilage and subchondral bone defects.
Methods: Specimen Preparation: One-centimeter sections were cut from twenty cadaver knees and ankles, milled to a thickness of 6.50 mm +/- 0.02 mm, and potted into aluminum loading platens.
Loading: Texture correlation is dependent on comparing high quality contact radiographs of loaded and unloaded specimens. Samples were secured to a loading frame, constrained between plexiglas plates inside a contact radiography unit, and axially loaded to 0, 225, and 450N. Images were captured on Kodak Industrex M film. Specimens were loaded intact, and with three increasing full thickness cartilage defects and three increasing circular subchondral defects. Defects were 10%, 20%, and 30% of the sagittal diameter of the distal tibia and of the coronal width of the medial plateau in the proximal tibia.
Strain Measurement: Digital images were made from X-rays using a light box and a CCD camera, distributing trabecular patterns over 106 pixels spanning 52,000 gray levels. Texture correlation measurements were performed with in-house code written in C, running on a Sun SPARCstation 10. Texture correlation compares digitized radiographs of a sample under load with its unloaded image to measure strains at high spatial resolution. Strain components were calculated at 2600 points within the juxtaarticular, epiphyseal and metaphyseal regions of the proximal and distal tibia. The points were kept in correspondence using an automated meshing procedure based on consistently measured anatomic landmarks.
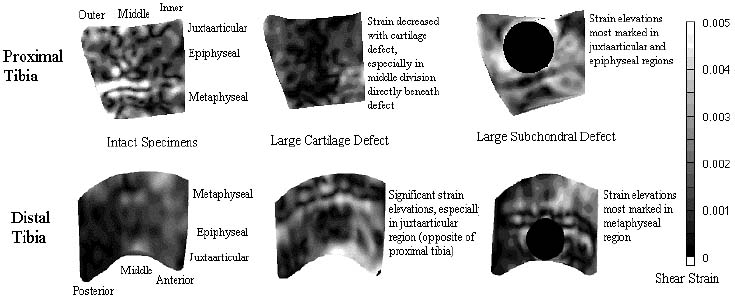
Statistical Analysis: We extracted the median shear strain value from divisions in the juxtaarticular (within 3 mm of cartilage), epiphyseal (between juxtaarticular region and physeal scar), and metaphyseal regions (above the physeal scar) to estimate the region of influence associated with each defect. Median values were averaged for all specimens on a region by region basis. Defect sample values were compared to intact sample values using one-way analysis of variance.
Results: Cartilage defects caused trabecular strain to increase in the distal tibia and decrease in the proximal tibia. This effect was most marked in the juxtaarticular region. Small defects had little effect on strain in the proximal tibia but caused significant strain elevations in the distal tibia. Subchondral bone defects caused strain elevations in both the distal and proximal tibia. The elevations were most marked in the metaphyseal region of the distal tibia and in the epiphyseal and juxtaarticular regions of the proximal tibia. Representative strain contour plots are displayed below.

Discussion: Strain has been shown to be an important physical signal regulating functional adaptation of bone. Direct measurement of strain in bone has been limited to cortical tissue, and trabecular strain measurements have relied on finite element models. We developed texture correlation to directly measure strain in trabecular bone, eliminating structural and material assumptions inherent to finite element analysis. In the intact samples of the proximal and distal tibia, strain distribution was relatively homogenous throughout the specimens. Interestingly, cartilage defects caused sharp strain elevations in the distal tibia and diametrically opposite decreases in shear strain in the proximal tibia. Subchondral bone defects caused strain elevations in both the distal and proximal tibia but in different regions. While concentrating strain elevations in the juxtaarticular and epiphyseal regions in the proximal tibia, subchondral defects caused strain elevations in the metaphyseal region of the distal tibia.
Cartilage, calcified cartilage, the subchondral plate, and subchondral bone form a complex, composite periarticular structure, designed to conduct loads from the articular surface to the diaphysis. Previous researchers have demonstrated structural changes in the subchondral tissues can lead to destruction of the adjacent cartilage. Strain changes, resulting from intraarticular injuries, may drive a pathological mechanical response throughout the trabecular bone, which may be instrumental in progression to post-traumatic arthritis. Differences in strain patterns resulting from subchondral and chondral defects may partially explain the markedly different clinical courses seen with intraarticular fractures of the knee and ankle.
Texture correlation is a new and powerful tool that allows direct measure-ment of strain in trabecular bone. However, our model is two-dimensional, and loads are applied for an extended period (95 seconds) to enhance image quality. These limitations alter mechanical and temporal boundary conditions. However, we feel we have measured realistic strain values and characterized relative effects on how cartilage and subchondral bone defects affect strain patterns in the proximal and distal tibia.