Session VI - Fracture Repair
Mechanical Strength of Fracture Callus in Osteopenic Bone at Different Phases of Healing
Donna L. Wheeler; Elizabeth J. Eschbach; Megan J. Montfort; Pramod Maheshwari; Sean W. McLouglin, PhD, University of Florida, Gainesville, FL; Oregon Health Sciences University, Portland, OR
Introduction: The incidence of osteoporotic-related fractures is established; however, very little basic science or clinical research has been conducted documenting the effects of established osteopenia on fracture healing. Strength and stiffness of fractured bone have been shown to increase non-linearly over the course of healing(1). Bone metabolism affects the mechanistic and temporal aspects of fracture repair, yet the specific effects of estrogen-deficiency and fracture healing are poorly documented. We hypothesized that ovariectiomized (OVX) estrogen-deficient rats would exhibit delayed callus formation and callus remodeling of midshaft femoral fractures when compared to SHAM animals, resulting in loss of mechanical strength at the fracture site. Therefore, the study objectives were to quantify and compare peak bending force and stiffness of fractured femurs during healing in OVX and SHAM-operated rats.
Materials and Methods: Sixty-six female Sprague-Dawley rats, 3 months old and weighing 250g, were randomly divided into two treatment groups (OVX and SHAM) and 4 euthanasia points based on fracture healing time (0, 4, 6, and 8 weeks). Bilateral OVX or SHAM procedures were performed on anesthetized animals. OVX animals were pair-fed based on the food consumption by SHAM rats. Four months following OVX, the 0 week animals were sacrificed to document intact bone biomechanics. The remaining animals were anesthetized and received bilateral femur fractures(2). Using sterile technique, two millimeter skin incisions were made over the antero-lateral greater trochanter and distal condyle. A 1.125 mm terminally threaded pin (Synthes, USA, Paoli, PA) was inserted proximally using a variable-speed wire driver (Hall Surgical, Warsaw, IN). Both cortices were engaged by the threaded portion on the pin. A jig was used to ensure parallel placement of the distal pin. A custom machined polymer external fixator was attached to the fixator pins and secured with set screws. After fixator placement, the femur was fractured midway between the proximal and distal pins using custom hand-operated pliers producing a 3-point bending load to the femur(3). Radiographs were taken immediately after fracture and at sacrifice with the animal in the prone position. Pin placement, fracture alignment, and degree of comminution were radiographically scored. At sacrifice the femurs were harvested and external fixator removed. The femurs were tested in 4-point bending to failure using an Instron 8511 (Instron Corp., Canton, MA). The loading rate was 0.1 mm/sec to a stroke of 5 mm and a sampling rate of 20 Hz. Raw load and displacement data were recorded and femoral stiffness calculated (slope of load/displacement curve). Data were analyzed using a 2-way analysis of variance (ANOVA) followed by a Duncan's multiple comparison procedure looking at the effects of treatment (OVX and SHAM) and healing time ( 0, 4, 6, and 8 weeks). Statistics were run at a significance level of p=0.05 and power of 80% using SAS software (SAS Inc., Cary NC).
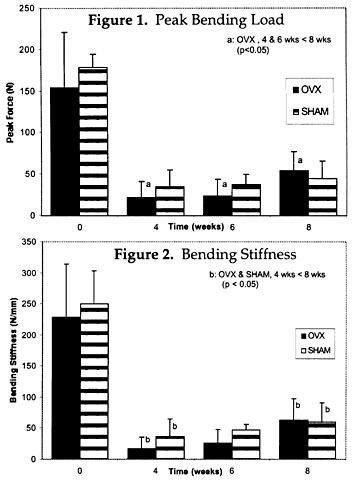
Results: The peak bending load of the healing fractured femurs in the OVX and SHAM was not significantly different. The peak bending load for the OVX animals at 4 and 6 weeks was significantly lower than the peak load at 8 weeks (p<0.05), whereas no difference was found for the SHAM animals (Fig 1). Both SHAM and OVX animals had greater bending stiffness of the healing fractured femur after 8 weeks of healing than at 4 weeks (p<0.05) (Fig 2).
Discussion: Our hypothesis that ovariectiomy-induced osteopenia would have an adverse healing effect upon midshaft femoral fractures was proven by this study. Although there was no statistical difference in the peak load or stiffness of the healing callus in OVX animals compared to SHAM animals, the rate of healing was delayed in the OVX animals with a significant increase in failure loads recorded at 8 weeks when compared to 4 and 6 weeks. Our results are consistent with those of others investigating fracture healing in OVX rats (4,5,6). In previous studies, rats received femur fracture fixed by intramedullary pinning 6 weeks following OVX . The fractures in our study were fixed with external fixators, which are less stable than intramedullary pinning, and fractures were induced 16 weeks following OVX. The reduction of systemic estrogen with OVX induces a period of high bone turnover and accelerated bone loss due to formation and resorption imbalance(7). The temporal relationship between OVX and fracture would affect the fracture healing cascade and the mechanical strength of the healing fracture. Hill et al.(4) studied fracture healing in OVX rats and found impaired healing after 21 days. Impaired fracture healing was quantified by breaking torque normalized to body weight, area of callus and trabecular density of bony callus. Similarly, Walsh et al.(6) investigated the effects of OVX on fracture healing using a rat model. Significant differences were found at 4 weeks between the peak uniaxial tensile loads and peak 4-point bending loads and stiffness betweem SHAM and OVX rats, but no differences were found at 2 or 6 weeks. Sherman-Brown et al.(5) investigated the effects of estrogen treatment on fracture healing of OVX rats and found significant improvement in healing in the treated rats. In spite of study inter-design inconsistencies, our study confirms the results of others. Fracture healing is impaired in an estrogen-deplete and/or osteopenic state. More research is needed in this area to elucidate our findings.

Acknowledgements: The authors would like to thank Synthes USA and Hall Surgical for donation of fixation pins and instruments. The financial support of the Orthopaedic Trauma Association has made this work possible.
References: (1) Acta Orthop Scand 1981; 52:605-613; (2) J Orthop Res 1984; 2:97-101; (3) J Dent Res 1979; 58:1093-1096; (4) Trans ORS 1995; 41:230; (5) Trans ORS 1997; 43:256; (6) Clin Orthop 1997; 342:218-227; (7) Bone 1986; 7:119-123.