Session VII - Foot / Ankle
Sat., 10/12/13 Foot/Ankle, PAPER #83, 10:58 am OTA 2013
Does Syndesmotic Injury Have a Negative Effect on Functional Outcomes? A Multicenter Prospective Evaluation
Jody Litrenta, MD1; Paul Tornetta, III, MD1; Laura S. Phieffer, MD2; Clifford Jones, MD3;
Janos P. Ertl, MD4; Brian H. Mullis, MD4; Kenneth A. Egol, MD5; Michael J. Gardner, MD6; William M. Ricci, MD6; David C. Teague, MD7; William J. Ertl, MD7; Cory A. Collinge, MD8;
Ross K. Leighton, MD9;
1Boston University Medical Center, Boston, Massachusetts, USA;
2Ohio State University Medical Center, Columbus, Ohio, USA;
3Orthopaedic Associates of Michigan, Grand Rapid, Michigan, USA;
4Indiana University, Indianapolis, Indiana, USA;
5NYU Hospital for Joint Diseases, New York, New York, USA;
6Barnes-Jewish Hospital, St. Louis, Missouri, USA;
7University of Oklahoma, Oklahoma City, Oklahoma, USA;
8Orthopedic Specialty Associates, Fort Worth, Texas, USA;
9Dalhousie University, Halifax, Nova Scotia, Canada
Background/Purpose: A negative prognosis has been reported for indirect ankle fractures with associated syndesmotic disruption as compared to those without syndesmotic injury. However, no report has separated Weber C from B injuries as a confounding variable. Ideally, this factor should be eliminated from the analysis to truly understand the effect of syndesmotic injury. Our purpose was to evaluate the effect of syndesmotic disruption on the functional outcomes of Weber B, SE4 (supination external rotation) ankle fractures treated surgically.
Methods: We performed a prospective multicenter evaluation of 242 patients (136 women, 106 men) with Weber B SE4 ankle fractures treated surgically. The average age was 46 years (range, 18-83). 81 (35%) of these patients had intraoperatively confirmed syndesmotic instability after fibular fixation and were reduced and fixed with syndesmotic screws. Outcomes evaluated at 6 weeks and 3, 6, 9, and 12 months included Short Musculoskeletal Function Assessment (SMFA), Bother Index, and American Orthopaedic Foot & Ankle Society (AOFAS) scores as well as symptomatic hardware and peroneal tendon discomfort. Statistical analysis was done using a mixed linear regression analysis using adjusted means with Tukey’s method to account for repeated measures by a PhD statistician for functional outcomes to evaluate the recovery curve of the two groups, and for gender and race. T tests and χ2 were used for other variables at the final 1-year outcomes.
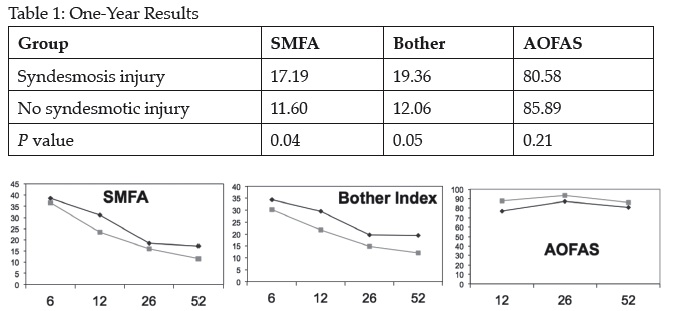
Results: The adjusted means regression analyses demonstrated that patients without a syndesmotic injury had better SMFA scores at 12 weeks (P = 0.02), but not at 6, 26, or 52 weeks (P = 0.76, 0.73, 0.32). No syndesmotic injury also resulted in statistically better scores for the AOFAS (P = 0.0006) and trended toward better results for the Bother Index (P = 0.07). Men had better results than women for all outcomes: SMFA (P = 0.002), Bother Index (P = 0.008), and AOFAS (P = 0.0006). Race was not a significant factor for any score. Isolated analysis of the 1-year results revealed a difference in the SMFA and Bother Index, but not the AOFAS (Table). At 9 to 12 months, hardware was symptomatic in 17% of patients with and 10% of those without syndesmotic fixation (P = 0.28), and peroneal symptoms present in 14% and 8%, respectively (P = 0.24).

Conclusion: Syndesmotic instability in association with Weber B, SE4 ankle fractures had worse outcomes at 1 year using the SMFA and bother indices. The difference was at the limit of clinical significance (1/2 standard deviation). Additionally, mixed linear regression over time demonstrated better results for the SMFA (only at 6 weeks) and the AOFAS with the Bother Index just outside of statistical significance. The most consistent finding, however, was better outcomes for men for all measures at all time points. Syndesmotic injury has a slightly detrimental effect on outcomes of surgically treated Weber B SE4 fractures.
Alphabetical Disclosure Listing
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.