Sat., 10/6/12 General Interest, PAPER #98, 1:27 pm OTA-2012
Duration of Fracture Fixation Surgery in Multitrauma Patients
Christopher E. Mutty, MD; Lars M. Qvick, MD; Mark J. Anders, MD;
Cathy M. Buyea, MS; Lawrence B. Bone, MD;
State University of New York at Buffalo, Erie County Medical Center, Buffalo, New York, USA
Purpose: Early orthopaedic surgery in the multiply injured patient has been reported to have a safe upper limit of 2 hours. A review of the literature reveals evidence of a 6-hour limit; however, no evidence was found for the 2-hour time constraint. We hypothesized that length of early surgery time would not be associated with patient outcome. Standard of care at our institution requires ongoing resuscitation and careful monitoring of patient status for surgery to continue as long as the patient is stable.
Methods: University IRB approval was obtained and a report was requested from the institutional trauma database of all multitrauma patients presenting with an ISS of ≥18 from January 2007 to May 2011. This report contained 1690 patients with 232 orthopaedic injuries; of these, 187 had long bone injuries and were included in the analyses. Data were analyzed with independent t tests for continuous variables and χ2 or Fisher exact test for categorical. Logistic regression analysis was used to determine predictors of mortality and acute respiratory distress syndrome (ARDS). A power analysis revealed that with alpha at 0.05 and 153 cases, we could determine a difference in odds of 1.5 with 80% power.
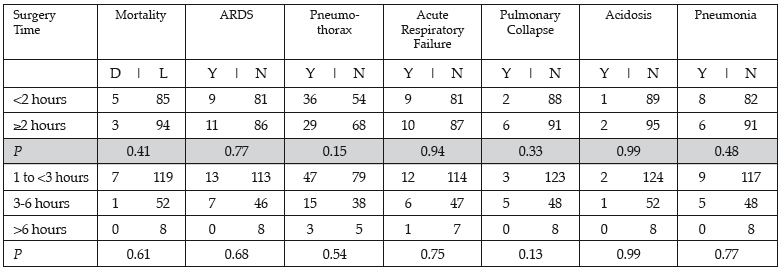
Results: There were no significant associations found with total minutes of surgery and pulmonary complications nor were there any significant associations found with surgery as a categorical value (<2, ≥2 hours; 1 to <3, 3-6, >6). Length of surgery was not a significant predictor or mortality (P = 0.45) or ARDS (P = 0.75).
Conclusion: This series of patients suggests duration of surgery alone need not limit early orthopaedic trauma procedures in the multiply injured patient.

Alphabetical Disclosure Listing (808K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.