Sat., 10/6/12 Femur/Tibial Fx/Knee Injuries, PAPER #68, 8:00 am OTA-2012
How High Can You Go: Retrograde Nailing of Proximal Femur Fractures
Lisa K. Cannada, MD; Kevin M. Kuhn, MD; J. Tracy Watson, MD;
Southeast Fracture Consortium;
Saint Louis University Hospital, Saint Louis, Missouri, USA
Purpose: Retrograde nailing (RGN) has been used for select indications in femoral shaft fractures. However, fractures in the proximal one-third have been considered “off limits” for RGN with increased complication rates anticipated. There are no data supporting recommendations on how proximal is too proximal for RGN. We describe a proximal segment capture ratio. It is our premise that a smaller capture ratio represents a very proximal fracture with less nail capture and thus will result in a higher rate of malunion/nonunion.
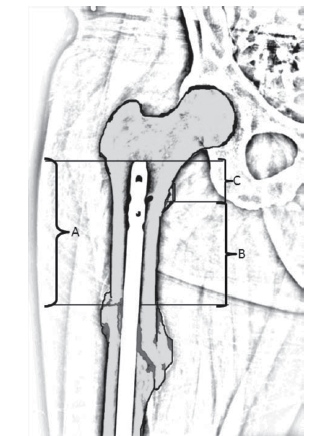
Methods: At six Level I trauma centers, skeletally mature patients with femur fractures within the proximal one-third of the femur treated with retrograde intramedullary nails were included. Clinical records were reviewed and data obtained and compared with regard to demographic information, comorbidities, associated injuries, operative details, time to union, time to full weight bearing, radiographic outcomes, complications, and need for secondary procedures. To evaluate RGN of proximal fractures, we describe a proximal segment capture ratio (PSCR). This ratio is determined by the length from the top of nail to fracture location (A) and the distance from the lesser trochanter (LT) to fracture site (B). The PSCR is a ratio of the amount of nail above the lesser trochanter (C) (see figure). Statistical analysis was completed using descriptive statistics, χ2 for nominal variables, and t tests for continuous variables.
Results: There were 107 patients (59 males, 48 females) with RGN of proximal one-third femur fractures with adequate radiographic and clinical follow-up defined as radiographic union and/or full weight bearing. The average age of the patients was 33 years (range, 17-71) with an average ISS of 19 (range, 9-75). The average tip of nail to fracture measure (A) was 12 cm (range, 2.3-19). The average distance from the LT to fracture site (B) was 8 cm (range, 0.7-12). The average PSCR (C/A) ratio was 0.35 (range, 0.04-0.89). The average time to union increased in those fractures with comminution. The average time to full weight bearing was 10 weeks with an average follow up of 44.4 weeks. There were 2 nonunions and 3 malunions. Nine patients required secondary procedures: 4 dynamizations and 1 revision to a plate with bone graft, all of which went on to heal; 1 wound débridement, 1 shortening procedure, and 2 procedures for heterotopic ossification removal. There was no significant difference between a PSCR ratio of 0.3 or less and need for secondary procedures or time to full weight bearing (P >0.05). The occurrence of malunion was increased with OTA C-type fractures and overall time to union was increased (P <0.05).
Conclusion: We describe a proximal segment capture ratio to help determine a cut-off distance whereby the amount of nail above the fracture versus distance of the fracture below the LT could define indications for proximal femoral shaft RGN. A smaller ratio could indicate a less stable construct and potentially a higher rate of nonunion/malunion. In our study, a smaller (<0.3) PSCR was not associated with increased number of secondary procedures, nonunion, or malunion. A higher OTA classification (increased comminution) was predictive of malunion and increased time to union. Fractures with increased comminution, despite an adequate nail capture ratio, trended toward varus malreductions and subsequent malunion. In this study, the proximity of the fracture to the LT alone did not affect results. Using those guidelines, RGN is safe and effective for the treatment of supraisthmal femur fractures.

Alphabetical Disclosure Listing (808K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.