Fri., 10/5/12 Pelvis & Acetabulum, PAPER #61, 3:34 pm OTA-2012
Is Closed Reduction and Percutaneous Fixation of Type 3 Posterior Ring Injuries as Accurate as Open Reduction and Internal Fixation?
Adam Lindsay, MD1; Paul Tornetta, III, MD1; Amna Diwan, MD2; David C. Templeman, MD2;
1Boston University Medical Center, Boston, Massachusetts, USA;
2Hennepin County Medical Center, Minneapolis, Minnesota, USA
Background/Purpose: Type 3 posterior ring injuries are complete injuries and typically treated with reduction and internal fixation. While open treatment through a posterior approach allows for direct fracture reduction and is the gold standard, many surgeons are employing more percutaneous approaches. We compared closed reduction and percutaneous fixation (CRPP) versus open reduction and internal fixation (ORIF) of type 3 posterior ring injuries with the hypothesis that CRPP would be equivalent to ORIF in quality of reduction.
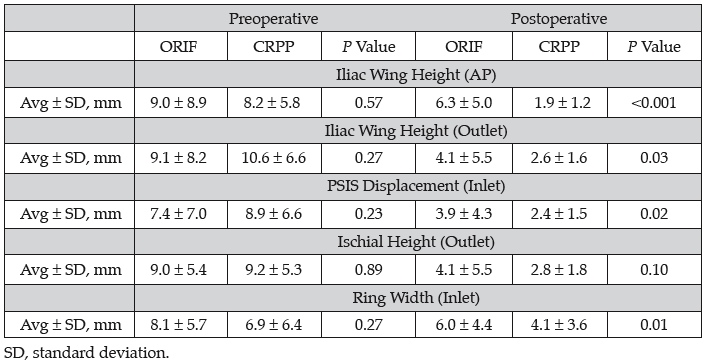
Methods: We reviewed 113 consecutive cases of unilateral unstable posterior ring injuries treated by two physicians in two different centers with iliosacral (IS) screws. Only true type 3 injuries were included. One surgeon routinely performed ORIF (n = 60); and the other, CRPP (n = 53). These two groups were compared. For all cases, demographic information, time to surgery, type of injury, and type of fixation was documented. Displacements were measured on the initial presentation and postoperative AP, inlet, and outlet views. These included the differences in the affected side from the normal side for: iliac wing height (AP, outlet), sacral height (AP), posterior superior iliac spine (PSIS) displacement (inlet), ischial height (AP, outlet), ring width (AP, inlet), and sacral width (inlet). These were compared with two-tailed t test assuming 0.05 for significance. CRPP Technique: A special traction setup was used that has not been previously described. The patient is positioned supine with a bump under the affected thorax and all the way to the edge of the table to allow easy placement of IS screws. The normal side foot is placed in a boot and the heel elevated to keep the knee in extension and locked there. Skeletal traction with ~20° to 30° of flexion is used on the affected side. A specially adapted hip positioning pad is locked to the table and abuts the affected thorax to prevent lateral displacement of the thorax when traction is applied. Under fluoroscopy, the posterior reduction can be titrated using traction as the unaffected leg acts as a post and the thorax pad prevents angulation of the body as the traction is applied to the affected side. Once the reduction is obtained, IS screws are placed. ORIF Technique: The patient is positioned prone on pads to allow free manipulation of the pelvis. A gluteus maximus–sparing approach was used in all cases. Clamps are used to directly reduce the posterior ring, augmented by fluoroscopy, and IS screws are placed.
Results: There were 53 patients treated with CRPP and 60 with ORIF during the study period. The ages (average, 36.9 years; range, 13-71), gender (62 female, 51 male), and ISS scores (average, 22.4; range, 4-59) did not differ between the groups. Initial displacements were also not different for the two groups (see table). The time to surgery was 4 days (range, 0-16) for the CRPP group and 5 (range, 0-36) for the ORIF group. Overall reduction quality was slightly better for the CRPP method for most parameters tested.

Conclusion: We compared the radiographs of two senior surgeons’ series of type 3 posterior pelvic injuries. One treated patients with standard ORIF and the other with CRPP. We found no differences in the initial displacements or demographics. The final reduction was statistically better with CRPP for most parameters; however, these differences likely have no clinical significance as the largest average difference was <5 mm. We conclude that CRPP done as described is as effective as the gold standard of prone ORIF in obtaining an accurate reduction of type 3 posterior pelvic ring injuries.
Alphabetical Disclosure Listing (808K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.