Fri., 10/5/12 Basic Science, PAPER #56, 1:52 pm OTA-2012
Short Segment Fixation of an L1 Compression Fracture: Four Versus Six Screws
Seth K. Williams, MD1; Robert P. Norton, MD1; Edward L. Milne, BSc2;
David N. Kaimrajh, MS2; Frank Eismont, MD1; Loren L. Latta, MD, PhD1,2;
1Department of Orthopaedics, University of Miami, Miami, Florida, USA;
2Max Biedermann Institute for Biomechanics, Miami Beach, Florida, USA
Purpose: The standard configuration for posterior short segment fixation involves pedicle screws placed above and below the fracture. However, loss of correction and hardware failure have been reported. In a series of 19 patients treated with short segment fixation, bending of the screws occurred in 6 patients, progressive kyphosis in 3 patients, and screw breakage in 1 patient. We hypothesize that placing pedicle screws at the level of the fracture posteriorly can improve the rigidity of the fixation.
Methods: 13 fresh-frozen human cadaveric spines (donor ages 20 to 60 years at time of death) were thawed and manually stripped of the paraspinal muscles, leaving the facet joints, capsules, and interspinous ligaments intact. The spines were separated at the T11-12 and L2-3 discs, leaving an intact segment from T12 through L2. Multiple holes were drilled in the L1 vertebral body in order to compromise its integrity and allow for the production of an isolated compression fracture. The vertebral column segment was then placed between metal plates and an incremental axial displacement was applied via an MTS MiniBionix II Model 858 servohydraulic testing machine until a compression fracture at L1 with a residual vertebral height loss of at least 50% was produced. Fluoroscopy was used to confirm a persistent height loss of at least 50% after the load was removed. The posterior elements were intact to visual inspection. The specimens were instrumented with 6-mm pedicle screws connected to 5.5-mm titanium rods. Selspot LED emitters were fixed to T12 and L2 to measure their motion in 6 degrees of freedom, from which the relative movements between T12 and L2 could be calculated. Uniaxial strain gauges were bonded to the rods to monitor longitudinal strain in the segment of rod between the T12 and L1 screws, and between the L1 and L2 screws. A 400-N follower preload was employed to simulate the stabilizing forces produced by paraspinal musculature. Specimens were cyclically loaded from 5 N·m extension to 5 N·m flexion, well within their elastic range. Two conditions were tested: (1) 4-screw construct: no screws at the L1 fractured body (4S); (2) 6-screw construct: screws at all levels (6S). The two groups were compared statistically by paired t test.
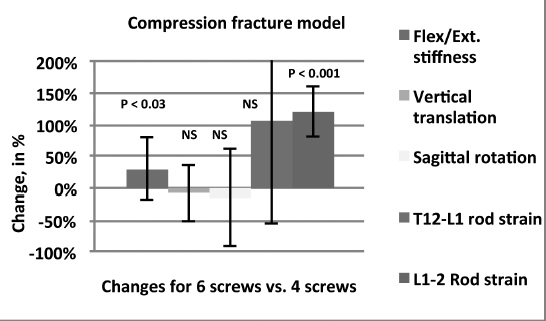
Results: The mean stiffness in flexion and extension was 13.42 N/mm with the 4-screw construct and 17.37 N/mm with the 6-screw construct (P <0.03). This represented a 31% increase in construct rigidity with the addition of the 2 screws in L1. Relative movement of T12 compared to L2 was evaluated in terms of vertical translation and sagittal rotation between both groups and no significant difference was found between the 4S and 6S constructs. With screws at L1, rod strain was significantly increased between L1-L2 (P <0.001), but not between T12-L1.
Discussion/Conclusion: The goal of placing additional screws in a fractured vertebral segment is to decrease the chance of mechanical failure with short segment constructs. The addition of screws at a fractured L1 segment increases the rigidity of fixation and places more of the load on the rods, but distributes the load over 50% more screws, which may decrease the potential for loosening of the screws. In a cadaveric L1 compression fracture model, a 6-screw construct with 2 screws in the injured vertebral body is biomechanically superior to a 4-screw construct that skips the injured body.
Figure The percent change from 4 screws to 6 screws for each parameter showed significant increases in structural stiffness and rod strains between L1 and T12.

Alphabetical Disclosure Listing (808K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.