Fri., 10/5/12 Basic Science, PAPER #52, 1:23 pm OTA-2012
•Local Bismuth Thiols Potentiate Antibiotics and Reduce Infection in a Contaminated Open Fracture Model
Jowan Penn-Barwell, MRCS1; Brett Baker, MSc, DC2; Joseph C. Wenke, PhD3;
1Royal Centre for Defence Medicine, Birmingham, United Kingdom;
2Microbion Corporation, Bozeman, Montana, USA;
3U.S. Army Institute of Surgical Research, Fort Sam Houston, Texas, USA
Purpose: This study screened different types and concentrations of Bismuth Thiols (BTs) for ability to potentiate systemic antibiotics in reducing infection in open fractures. We hypothesized that BTs would potentiate systemic antibiotics because BTs are known to reduce biofilms, one of the major barriers for antimicrobial success.
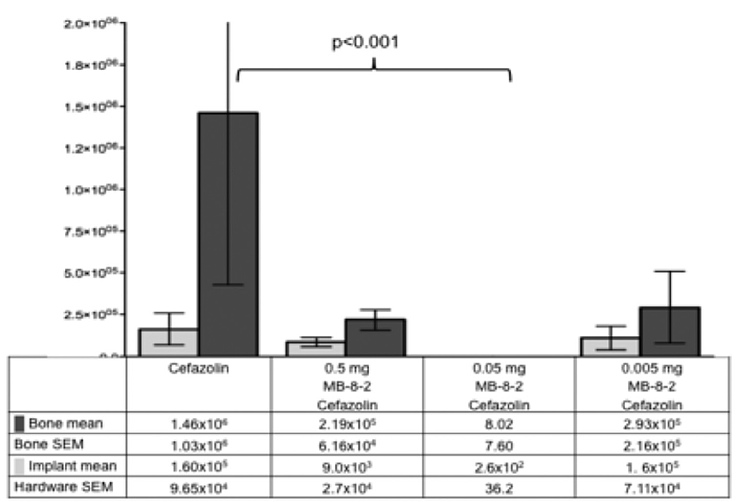
Methods: A segmental defect rat femur model contaminated with Staphylococcus aureus and treated with surgical débridement 6 hours after injury and 3 days of systemic cefazolin was used. BTs suspended in a hydrogel were added to the wound immediately after débridement. After 14 days, the bone and implant were harvested for microbiologic analysis. The principal outcome was the quantity of bacteria on the implant or bone.
Results: Of the 3 formulations of BTs screened, the formulation with the best profile of low toxicity and high antimicrobial effect was retested at a range of doses. A dose of 0.05 mg when combined with systemic cefazolin resulted in 0.02% of the bacterial quantity of the cefazolin only group (P <0.001), with the rate of detectable bacteria in wounds dropping from 60% to 10% (P = 0.0022), and no observable local and systemic toxic effects.

Conclusion: BTs at an appropriate dose can potentiate the effect of antibiotics at reducing infection rate and bacteria quantity.
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, Department of Defense, or the U.S. government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred. This work was performed at the U.S. Army Institute of Surgical Research. This study has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals.
Alphabetical Disclosure Listing (808K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.