Sat., 10/15/11 Recon, UE, Wrist & Hand, Paper #82, 3:30 pm OTA-2011
The Fate of Patients After a Staged Nonunion Procedure for Known Infection
Paul Tornetta, III, MD1; Kevin Dale, MD1; Clifford B. Jones, MD2; Brian H. Mullis, MD3;
Kenneth A. Egol, MD4; Elliot Robinson, MD5; Michael J. Bosse, MD5;
Andrew H. Schmidt, MD6; Robert A. Hymes, MD7;
1Boston University Medical Center, Boston, Massachusetts, USA
2Orthopaedic Associates of Michigan, Michigan State University, Grand Rapids, Michigan, USA
3Indiana University, Indianapolis, Indiana, USA;
4NYU Hospital for Joint Diseases, New York, New York, USA;
5Carolinas Medical Center, Charlotte, North Carolina, USA;
6Hennepin County Medical Center, Minneapolis, Minnesota, USA;
7Inova Fairfax Hospital, Fairfax, Virginia, USA
Purpose: Patients who had prior surgery and develop an infected nonunion typically undergo a staged reconstruction including multiple débridements, hardware removal, and antibiotics. The purpose of this study is to review a large series of patients who underwent staged procedures for the treatment of infected nonunions, highlighting the course of treatment and the ultimate result with respect to union and eradication of infection.
Methods: Patients treated for nonunion at 7 academic medical centers who were treated with a staged protocol for an infected nonunion were evaluated. The course of the patients was documented including the use of antibiotics, number of débridements, hardware treatment, dead space management, coverage, definitive surgery performed, and the outcome regarding infection and union.
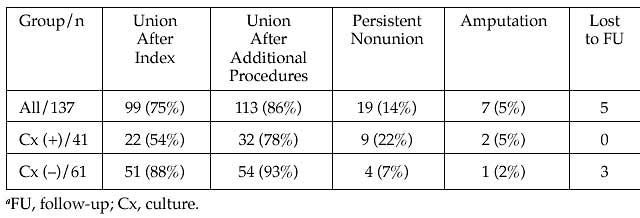
Results: There were 137 staged procedures for infected nonunions of the tibia (92), femur (25), humerus (8), or other (14) in 94 men and 43 women with an average age of 43.7 years. All patients had operative treatment of their initial fractures. 104 (76%) became infected after their initial operative procedure and 33 (24%) after an additional procedure. 26 different bacteria were cultured with methicillin-sensitive Staphylococcus aureus (MSSA) (25), methicillin-resistant Staphylococcus aureus (MRSA) (20), coagulase-negative Staphyloccus (15), Enterococcus (13), and Enterobacter (11) most common. 31% were polymicrobial. The initial hardware was removed (78), retained (25), or exchanged (10), with 2 patients treated initially with no internal hardware. Adjuvant defect management consisted of antibiotic beads (34), intramedullary nail (IM) (32), or spacer (13). Patients had an average of 2.9 débridements and received antibiotics for an average of 6.4 weeks prior to their definitive nonunion procedure. Definitive procedures for nonunion included IM nail (51), open reduction and internal fixation (48), bone grafting (16), Taylor Spatial Frame external fixation (10), and, in 4 patients in whom the infection was not resolved, amputation. Closure was primary (63), vacuum (15), free flap (25), or split-thickness skin graft (5). 65 patients (55%) were supplemented with osteobiologic products. 102 patients had cultures at the definitive procedure resulting in 41 (40%) positive cultures and 61 (60%) negative cultures. The C-reactive protein and erythrocyte sedimentation rate prior to the definitive procedure was 4.1 and 45 for those with (+) and 2.1 and 23.5 for those who had (–) cultures at the definitive procedure. The table below shows the results for all patients as well as the breakdown of those with (+) and (–) cultures at the time of definitive management. After union, 76 (67%) • The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). For full information, refer to page 411. 243 of the patients with hardware retained their hardware and 37 (33%) patients had their hardware removed.
Table: Outcomes for the Patients Based on Index Culture Results.

Conclusions: Even with multiple débridements and antibiotics, 41% of patients had positive cultures at the time of their definitive management, which led to a disparate union rate as compared to those with a negative culture. This large series of staged procedures for infected nonunions demonstrates the difficulty in eradicating the infection even with modern dead space management and multiple débridements. This information will be helpful in setting the expectations of patients with this complex problem.
Alphabetical Disclosure Listing (628K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.