Fri., 10/14/11 Knee, Foot & Ankle, Paper #63, 4:27 pm OTA-2011
Twenty-Year Follow Up of Conservatively Treated "Isolated" Posterior Malleolar Ankle Fractures: A Case Series
Christian Donken, MD1; A.J.F. Goorden1; Michael Verhofstad, PhD2;
Michael J. Edwards, PhD1; Cees van Laarhoven, PhD1;
1Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands;
2St. Elisabeth Hospital, Tilburg, Netherlands
Purpose: The present study shows the long-term results of 24 patients with isolated posterior malleolar fractures between 1985 and 1990.
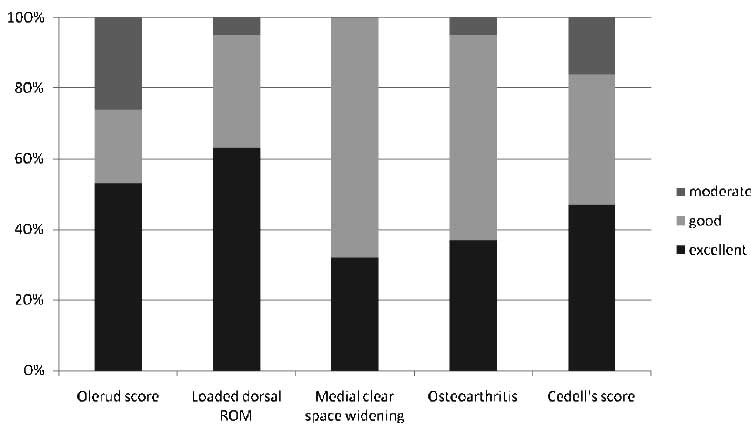
Methods: Outcome parameters used were (1) a functional outcome questionnaire (Olerud score), (2) physical examination (loaded dorsal range of motion [ROM]), (3) radiologic signs of instability (medial clear-space widening), (4) radiologic anatomic result (Cedell score), and (5) posttraumatic long-term damage (osteoarthritis).
Results: After a median of 20 years (range, 17-24 years), follow-up was achieved in 95% (n = 19) of living available patients. All patients were treated conservatively. The median Olerud score was 100 points, with 53% of the patients in the “excellent” result group. Excellent results were scored in 63% (loaded dorsal ROM), 32% (medial clear-space widening), 37% (osteoarthritis), and 47% (Cedell score). “Good” results were scored in 21% (Olerud score), 32% (loaded dorsal ROM), 68% (medial clear-space widening), 58% (osteoarthritis), and 37% (Cedell score). “Moderate” results were scored in 26% (Olerud score), 5% (loaded dorsal ROM), 5% (osteoarthritis), and 16% (Cedell score). Not 1 patient scored a “poor” result in any of the outcome parameters (Figure).

Conclusions: Conservative treatment of isolated posterior malleolar fractures resulted in good clinical and radiologic outcome in this series, with proof of little osteoarthritis at long-term follow-up.
Alphabetical Disclosure Listing (628K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.