Fri., 10/15/10 Foot & Ankle, Paper #53, 11:54 am OTA-2010
Summed Scores for Hindfoot and Ankle Trauma: What Do They Really Tell Us and How Many Do We Need?
Paul Tornetta, III, MD1; Rabah Qadir, MD1; Roy Sanders, MD2;
1Boston University Medical Center, Boston, Massachusetts, USA;
2Tampa General Hospital, Tampa, Florida, USA
Background: Recent emphasis on evidence-based medicine has resulted in more “patient-based” summed scores being used to report functional outcomes without reporting of the component scores. Issues of floor and ceiling effects are widely appreciated, but little is known about the effect of each of the component scores on the overall summed score in hindfoot and ankle injury.
Purpose: The purpose of this study was to determine which subscores contributed to the overall variation in summed scores, paying particular attention to pain, and to evaluate the correlation of multiple scoring systems (Short Form 36 physical component score [SF-36 PCS], American Orthopaedic Foot & Ankle Society [AOFAS], and Maryland) for calcaneus fractures, pilon fractures, and ankle fusions. Additionally, the correlation of range of motion with summed outcomes was examined.
Materials: 46 pilon fractures, 44 calcaneus fractures, and 54 ankle fusions were evaluated at greater than 2 years after injury with the SF-36, AOFAS, and Maryland scoring systems. Correlations were made between the summed scores using a Pearson correlation matrix, and the percentage of the overall variation of the summed score that is accounted for by the answer to the pain question within each score was determined. For the SF-36, this is the bodily pain subscale, and for the AOFAS and Maryland it is a single question about pain. The correlation of ankle and subtalar motion for pilon and calcaneus fractures and subtalar motion for ankle fusions with the summed scores was determined and added to a regression analysis if significant.
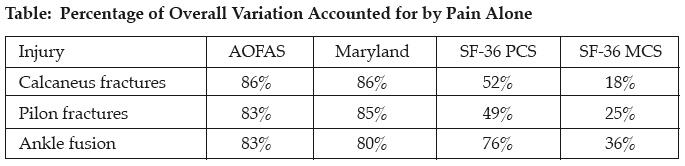
Results: For all subsets of patients, there was a strong statistical correlation between the SF-36 PCS, AOFAS, and Maryland scores (P < 0.001). The strongest correlation was between the AOFAS and the Maryland scores: calcaneus (r = 0.9565, P < 0.0001), pilon (r = 0.9463, P < 0.0001), and ankle fusion (r = 0.9436, P < 0.0001). The MCS (mental component score of the SF-36) also correlated with the other scores for the pilon and ankle fusion groups (P < 0.04), but not for the calcaneus group (r = 0.26). Ankle motion correlated with outcomes for the pilon fractures but not the calcaneus fractures. Subtalar motion correlated with outcomes for ankle fusions and pilon fractures, but not for calcaneal fractures. The overall summed scores were markedly affected by the pain score for all of the outcome scores examined. The individual subscale of pain accounted for as much as 86% of the overall variation. Even the SF-36 MCS was affected, with 18% to 36% of the overall variation being accounted for by pain (see table). Adding range of motion to the regression model did not account for more of the variation in outcome scores than did pain alone.

Discussion: Summed scores in evaluating outcomes for complex hindfoot and ankle injury and reconstruction show a high degree of correlation, particularly the AOFAS and Maryland scores. For all scores, pain is the dominant factor in the total variation of the scores. It represents more than 80% of the overall variation in the AOFAS and Maryland scores. Even the MCS is affected by the bodily pain subscale to a large degree.
Conclusions: Summed scores for complex hindfoot and ankle injury and reconstruction may not be needed in comparing outcomes as pain is the single dominant factor. We strongly recommend that when summed scores are used, that the individual components are reported so that surgeons may interpret the raw data. Future instruments may not need to be as burdensome as the current set of disease-specific measures as pain is the overriding constituent of the summed scores.
Alphabetical Disclosure Listing (292K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.