Fri., 10/9/09 Pilon/Foot & Ankle, Paper #63, 5:09 pm OTA-2009
The Preoperative Diagnosis of Infection in Nonunions
Charlton Stucken, MD (n); Dana Olszewski, MD (n); William Creevy, MD (n);
Paul Tornetta, III, MD (3,5A, 7-Smith &Nephew; 8-Exploramed);
Boston University Medical Center, Boston, Massachusetts, USA
Purpose: The surgical treatment of patients with a nonunion is changed in the face of infection. Prior to the surgical treatment of nonunions, in the absence of obvious infection, surgeons use a variety of methods to rule out quiescent infection. The patients at highest risk are those with a distant history of infection, those who have had prior surgery, and those who had sustained open fractures. The purpose of this study is to report on the utility of a standardized protocol to rule out infection in these high-risk patients, and to evaluate the efficacy of each component of the protocol.
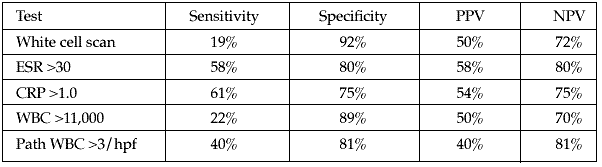
Methods: A single protocol of preoperative laboratory tests (white blood cell [WBC], C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]) and a combined white cell/sulfur colloid scan were performed in high-risk nonunion patients. Intraoperatively, patients had specimens sent to pathology for number of WBC/hpf (WBC count per high-powered field), and to microbiology for stat Gram stains with follow-up cultures. Infection was diagnosed based on positive intraoperative cultures, a finding of gross infection, or infection in the immediate postoperative period. Using infection as an end point, the sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively) for these tests were determined. Standard values were used as cutoffs for the blood tests (see table below). The white cell/colloid scans were read by radiologists and the report confirmed. Intraoperative values of > 3 WBC/hpf was used as an end point for the pathology specimens, and any bacteria on the Gram stain was considered positive. The use of a combination of risk factors (risk stratification method) was also evaluated.
Results: 93 patients with 95 nonunions and available records for the above protocol were surgically treated for nonunion. All patients were followed to union or infection as an outcome. There were 56 men and 37 women of mean age 46 years (range, 17-81) with 48 tibial, 27 femoral, 8 humeral, and 12 other nonunions. 30 of the 95 nonunions were ultimately diagnosed as being infected. The utility of the protocol components are seen in the table. No patient without frank pus had a positive intraoperative Gram stain.
Using a combination of the risk factors (elevated WBC, ESR, CRP and + scan), the PPV for 0, 1, 2, and 3 risk factors being positive were 22%, 36%, 57%, and 83%, respectively.

Eliminating the more expensive nuclear scan, leaving only WBC, ESR, and WBC, the PPV for 0, 1, 2, and 3 risk factors were 25%, 27%, 58%, and 100%.
Conclusions: In the face of prior surgery or history of open fracture, quiescent infection is common. None of the tests that we performed on this difficult population of patients was independently accurate. The high NPV of the CRP, ESR, and WBC/hpf seem to be the most beneficial independent components of the protocol. Using a risk stratification method, the likelihood of infection rose with each additional positive test. A combined white cell/sulfur colloid scan has a high specificity, but a very low sensitivity in revealing quiescent, chronic infection and does not seem cost-effective, even as part of a stratification scheme. We recommend simple blood tests and intraoperative WBC/hpf for the pre- and intraoperative workup of quiescent infection in high-risk nonunions.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant