Fri., 10/9/09 Pilon/Foot & Ankle, Paper #57, 4:23 pm OTA-2009
Twenty-One-Year Follow-Up of Supination-Eversion (SE) II-IV Ankle Fractures: A Retrospective Cohort Study
Christian Donken, MD1 (n); Michiel Verhofstad, MD1 (n); Cees van Laarhoven, MD2 (n);
1Saint Elisabeth Hospital, Tilburg, The Netherlands;
2University Medical Centre Saint Radboud, Nijmegen, The Netherlands
Purpose: We conducted an evaluation of long-term results after protocolled treatment of SE II-IV (AO type B) ankle fractures, in which “stable” supination-eversion (SE II and III) fractures were treated conservatively and “unstable” SE IV injuries treated by open reduction and internal fixation.
Methods: All 276 patients in our hospital with an SE II through IV ankle fracture between 1985 and 1990 were treated according to a strict treatment protocol. Stable fractures with tibiotalar congruity were treated conservatively and unstable fractures with incongruity were treated operatively. At follow-up, all patients were approached to participate in this study. Outcome parameters used were: (1) a functional outcome questionnaire (Olerud score), (2) physical evaluation (loaded dorsal range of motion [ROM]), (3) radiological signs of instability (medial clear-space widening), (4) radiological anatomical result (Cedell score), and (5) posttraumatic long-term damage (osteoarthritis).
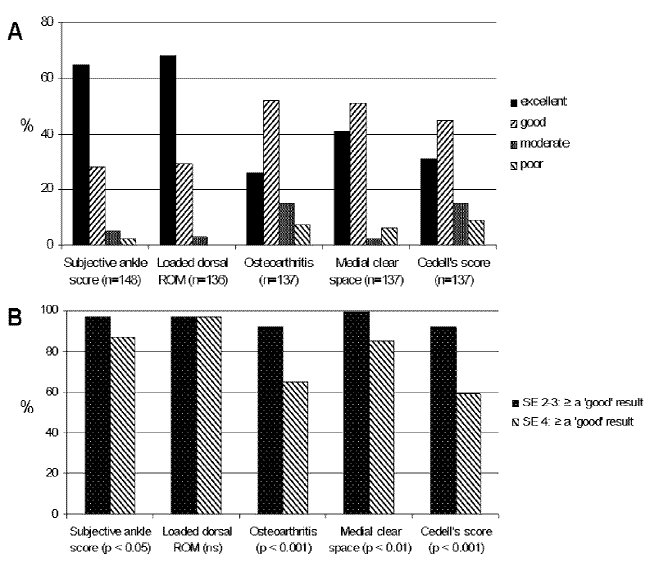
Results: A median of 21 years follow-up in 89% (n = 148) of living patients was achieved. 80 patients (54%) had an SE II or III injury, of whom 26 (33%) received osteosynthesis despite the treatment protocol. 68 patients (46%) had sustained an SE IV injury, of whom 60 (90%) were operated. Overall, “excellent” or “good” results were found in 76% to 97% (Figure A, below). SE IV injuries performed significantly worse than SE II or III injuries (Figure B).
Conclusion: The very long-term overall results of the stratified surgical treatment of SE II through IV ankle fractures gave an excellent to good result in 76% to 97% of patients and therefore seems justified. However, whether the unfavorable outcome of SE IV fractures is merely due to the more extensive damage (bony, cartilage, and ligaments) or due to the additional operative trauma cannot be determined in this study.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant