Sat., 10/18/08 Upper Extremity, Paper #68, 4:03 pm OTA-2008
Functional Outcomes for Three- and Four-Part Proximal Humerus Fractures Treated with a Locking Plate or Hemiarthroplasty
Ariana DeMers, DO (n); Robert J. French (n); Bradley Jelen, DO (n); Melanie Watts, ATC (n);
Palaniswamy Vijay, PhD (n);
OrthoIndy, Indianapolis, Indiana, USA
Purpose: With the advent of the locking precontoured proximal humerus plate, fixation of three- and four-part proximal humerus fractures has become an attractive option. The purpose of this study was to report the surgical and functional outcomes of locked plate fixation verses hemiarthroplasty in these fractures.
Methods: This study included 56 patients with three-part and four-part proximal humerus fractures from 2002-2005 with a mean follow-up time of 35 months. The mean patient age was 58.8 years for the open reduction and internal fixation (ORIF) group (n = 42) and 68.7 years for hemiarthroplasty group (n = 14). IRB approval was obtained and functional outcomes questionnaires were sent out with an invitation to return to the office for a physical examination. Range of motion (ROM), the Constant score, the American Shoulder and Elbow Surgeons score (ASES), the Simple Shoulder Test (SST), the Euroqol EQ-50, a visual analog pain scale (VAS), and the UCLA shoulder score were used to evaluate the patients.
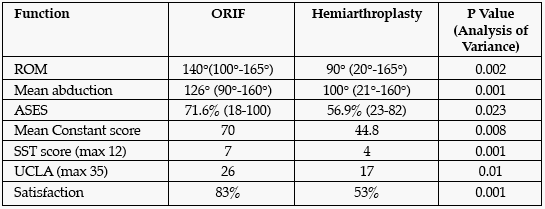
Results: Radiologic review of the ORIF group showed union in 41 patients and osteonecrosis in 1 patient who underwent subsequent hemiarthroplasty. Plate removal was performed in 1 patient after 3 months from the initial surgery, because of impingement symptoms. The scores of EuroQol EQ-5D (73 ± 24 vs 63.2 ± 21, P = 0.169) and VAS (2 ± 2 vs 3.1 ± 2.8, P = 0.135) were not statistically significant. Validated functional scores are given below.

Conclusion and Significance: Open reduction with internal fixation of three- and four-part proximal humerus fractures using a locking proximal humerus plate provides stable fixation that encourages bony healing and allows for early ROM and also better functional results and satisfaction when compared to hemiarthroplasty.
If noted, the author indicates something of value received. The codes are identified as a-research or institutional support; b-miscellaneous funding; c-royalties; d-stock options; e-consultant or employee; n-no conflicts disclosed, and *disclosure not available at time of printing.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.