Fri., 10/17/08 Basic Science, Paper #20, 10:04 am OTA-2008
2.5D Ultrasound for Measuring Leg Geometry
Peter Keppler, MD1 (n); Volker Sauer1 (n); Jean Baptiste Pinzuti2 (e-Engineer of Aesculap
Company); Josef Kozak2 (e-Engineer of Aexculap Company); Florian Gebhard, PhD1 (n);
1Department of Traumatology, University of Ulm, Ulm, Germany
2B. Braun / Aesculap AG, Tuttlingen, Germany
Purpose: 2.5D ultrasound has proven to be very useful in the analysis of the leg geometry pre- and postoperatively. Reasons why 2.5D ultrasound is not routinely used in the clinical setup is the necessity of special navigation equipment and a controversial accuracy and reproducibility of this technique. Therefore we integrated an ultrasound machine in a widespread-use navigation system and evaluated accuracy and inter- and intraobserver variability. The hypothesis was that 2.5D ultrasound is as good as CT and radiography in torsion, length, and axis measurements of the lower leg.
Methods: We combined an ultrasound machine (TELEMED) with a navigation system (Orthopilot). On the calibrated 5-MHz ultrasound probe, we fixed active markers on a circular manner, to guarantee visibility in every position of the space. With a specially developed software, all osseous landmarks seen by ultrasound can be saved with the coordinates of the ultrasound picture. After saving the picture, the mode of the ultrasound probe changes and the probe becomes a pointer on the screen. This mode makes it possible to mark the osseous landmarks on the ultrasound picture immediately, even under sterile conditions. We verified the accuracy of the system with a specially designed leg model. Every measurement was repeated five times by the same investigator. The torsion of the femur, tibia, and leg was measured in 2° steps from –40° to +40° and the mechanical leg axis in 1° steps from 20° varus to 20° valgus. Due to the design of the bone model, we were able to measure three femur lengths (468 mm, 510 mm, and 533mm), three tibia lengths (296 mm, 338 mm, and 381 mm), and 21 different leg lengths depending on the mechanical leg axis ranging from 835 mm to 848 mm. The reproducibility was verified on 25 healthy volunteers with a mean age of 25 years (range, 18-45 years). Two independent investigators measured the length and torsion of the femur, tibia, and the whole leg and the mechanical leg axis at 2 different days.
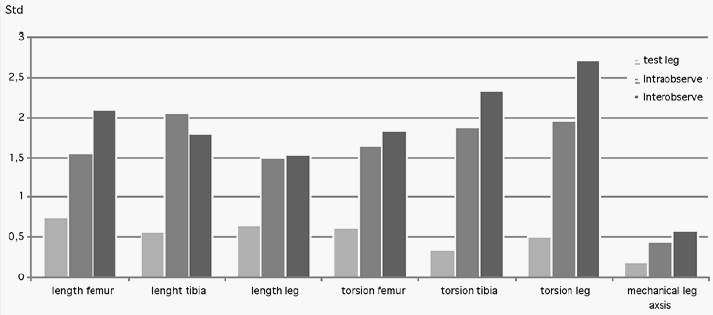
Results: Accuracy measurements on the model: The maximum deviation in all 40 length measurements was 1.5 mm, the maximum deviation of all 120 torsion measurements was 1.5°, and the maximum deviation of 41 leg axis measurements was 0.5°. The maximum error of intra- and interobserver variability in 25 healthy volunteers of the femur length was 3 mm and 6 mm, tibia length 6 mm and 6 mm, leg length 5 mm and 5 mm, femur torsion 5° and 7°, tibia torsion 5° and 7°, leg torsion 6° and 8°, and leg axis 1° and 1.5°. The standard deviations of all measurements calculated according to the mixed model method are shown in the figure below. Standard deviations of all measurements (N >1000) calculated according to the mixed model method.

Conclusion: According to the literature, the accuracy and reproducibility of this system is as good as CT scans and long-standing radiographs. Today the Orthopilot system is the leading navigation system in orthopaedic surgery. To perform a 2.5D ultrasound measurement with this system, only the ultrasound tool and the software is necessary. Further possible applications are the intraoperative measurement of the leg geometry after high tibial osteotomy, intramedullary nailing, or the intraoperative control of the real leg length after total hip implantation.
If noted, the author indicates something of value received. The codes are identified as a-research or institutional support; b-miscellaneous funding; c-royalties; d-stock options; e-consultant or employee; n-no conflicts disclosed, and *disclosure not available at time of printing.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.