Fri., 10/19/07 Tibia, Paper #21, 10:30 am OTA-2007
A Randomized Trial of Reamed versus Non-Reamed Intramedullary Nail Insertion on Rates of Reoperation in Patients with Fractures of the Tibia
Sprint Study Trial Co-Principal Investigators:
Mohit Bhandari*; Gordon Guyatt*; David W. Sanders#; Emil H. Schemitsch^; Marc Swiontkowski**; Paul Tornetta III##; Stephen Walter*
Site Investigators:
David W. Sanders1; Hans Kreder2; Yves Laflamme3; Emil H. Schemitsch4; Robert McCormack5; Lawrence X. Webb6; Paul Tornetta III7; William Todd Obremskey8; Heather A. Vallier9; Scott Mandel10; Peter A. Cole11; David Seligson12; Kevin J. Coupe13; Mark J. Anders14; Stephen J. Augustine15; J. Carel Goslings16; David C. Teague17; Donald W. Weber18; Kyle J. Jeray19; James N. Powell20; Stephen Connolly21; Thomas Ellis22; Theodore Miclau III23; David Karges24; Dennis J. Beck25; David Puskas26; Kenneth A. Egol27; Eugene K. Wai28
Introduction: Surgeons agree on the benefits of intramedullary nailing of tibial shaft fractures. Two alternative nailing approaches, with and without reaming, each have a compelling biological rationale and strong proponents. The SPRINT primary outcome study aimed to assess the impact of reamed versus unreamed intramedullary nailing on rates of reoperation in patients with open and closed fractures of the tibial shaft. Pending several decisions regarding additional evaluations needed for publication, the groups remain concealed at the time of writing, so as to maintain a fully concealed analysis. The groups will be referred to as A and B in this abstract.
Methods:
Design: The Study to Prospectively evaluate Reamed Intramedullary Nails in Tibial fractures (SPRINT) was a multicenter, randomized trial including 29 clinical sites. Over a 5-year period, SPRINT enrolled 1339 skeletally mature patients with open or closed fractures of the tibial shaft amenable to surgical treatment with an intramedullary nail. Patients, outcome assessors, and data analysts were blinded to treatment allocation. Perioperative care was standardized, and reoperations before 6 months were not permitted unless there was critical bone loss (>1 cm, >50% bone). Patients were followed at discharge, 2 weeks postdischarge, and at 3, 6, 9, and 12 months postsurgery. A blinded committee adjudicated all outcomes.
Intervention: Patients received a statically locked intramedullary nail with either reamed or unreamed insertion. No reaming was permitted in the unreamed group.
Main Outcome Measures: The primary outcome was reoperation to promote healing, treat infection, or preserve the limb (fasciotomy for compartment syndrome after nailing). The primary outcome was a composite comprising the following reoperations: bone grafts, implant exchanges, dynamizations, and autodynamizations in patients with fracture gaps <1 cm after intramedullary nail insertion. Infections and fasciotomies were considered events irrespective of the fracture gap. We planned a priori to conduct a subgroup analysis of outcomes in patients with open and closed fractures. Our sample size calculations required 1200 patients followed for 1 year.
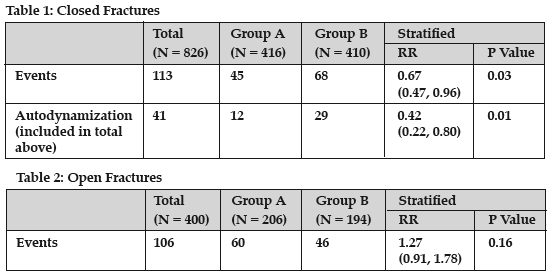
Results: Among 1339 enrolled patients, 1226 patients were followed to 1 year. Across treatment groups, patients did not differ in age, gender, and closed and open fracture types (I-IIIB). The overall event rate was 17.8% (13.7% closed, 27% open fractures). A significant subgroup interaction effect in patients with open versus closed fractures (P = 0.01) mandated a separate analysis for each subgroup. In 826 patients with closed fractures (Table 1), group A reduced the risk of an event by 33% (95% confidence interval: 4%-53%, P = 0.03). This treatment effect was largely driven by differential autodynamization rates (relative risk [RR]: 0.42, P = 0.01). Among 400 patients with open fractures (Table 2), group A trended towards an increased risk of an event (RR: 1.26, P = 0.16).

Conclusions: SPRINT investigators remain blinded to treatment allocation (this will be revealed in upcoming weeks). Based upon our blinded results, we identified an overall incidence of revisions smaller than reported in previous studies. Possible reasons for the overall lower event rates in SPRINT are as follows: (1) standardization of surgical and postsurgical care results in superior care among the SPRINT centers and surgeons and (2) proscription of surgery until after 6 months. The treatment effect in closed fractures favoring group A was driven by the differential rates of dynamization (and especially autodynamization) between groups. Autodynamization represents the least important measure of our composite end point as it did not require a surgery to gain union. Optimization of perioperative care and avoiding premature reoperation may substantially decrease the need for reoperation in tibial fracture patients.
This Study was funded by Research Grants from the Canadian Institutes of Health Research # MCT-38140 [PI: G. Guyatt], National Institutes of Health NIAMS-072; R01 AR48529 [PI: M. Swiontkowski], Orthopaedic Research and Education Foundation [American Academy of Orthopaedic Surgeons [PI: P. Tornetta III], Orthopaedic Trauma Association [PI: M. Bhandari]. Smaller site specific grants were also obtained from Hamilton Health Sciences Research Grant [PI: M. Bhandari] and Zimmer [PI: M. Bhandari].
Dr. Bhandari is funded, in part, by a Canada Research Chair in Musculoskeletal Trauma, McMaster University.
If noted, the author indicates something of value received. The codes are identified as a-research or institutional support; b-miscellaneous funding; c-royalties; d-stock options; e-consultant or employee; n-no conflicts disclosed, and *disclosure not available at time of printing.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing.