Thurs., 10/18/07 Polytrauma, Paper #10, 2:40 pm OTA-2007
Long-Term Outcome Assessment after Trauma: Does Gender Play a Role?
Christian Probst, MD1 (n); Boris Zelle, MD2 (n); Martin Panzica, MD1 (n);
Ralf Lohse, MD3 (n); Nicola Alexander Sitarro, MD3 (n); Christian Krettek, MD1 (n);
Hans-Christoph Pape, MD2 (n);
1Department of Trauma, Hannover Medical School, Hannover, Germany;
2Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, USA;
3Hannover Re Insurances, Hannover, Germany
Hypothesis: In the acute phase after multiple trauma, women before menopause seem to be protected against severe posttraumatic inflammatory reactions resulting in multiple organ dysfunction syndrome and/or death by estrogens and other sexual hormones. We examined, in addition to a reduced mortality, women might also gain higher quality of life and better rehabilitation status in the long term.
Methods: We assessed 637 polytrauma patients between 3 and 60 years of age treated at our level I trauma center with a minimum of 10 years’ follow-up (mean 17 ± 5) after the trauma for their rehabilitation status and quality of life including Hannover Score for Polytrauma Outcome (HASPOC), Short Form 12 (SF-12), and other parameters. Patients answered a questionnaire and were physically examined by a surgeon. Mental health was assessed by the impact of event scale (IES) and the hospital anxiety and depression scale (HADS).
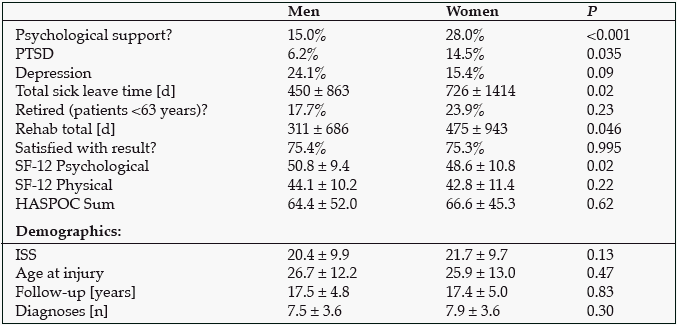
Results: Women showed a higher rate of posttraumatic stress disorder (PTSD) and use of psychological support, longer duration of rehabilitation, and longer sick leave time. Their quality of life was slightly but significantly lower, but the same rate of women as men felt well rehabilitated (see table).
Conclusion/Relevance: We conclude that even a long time after multiple trauma, women suffer more from the consequences than men. The rehabilitation of women might be adapted to their specific needs; especially, concomitant psychological treatment might be initiated earlier.
If noted, the author indicates something of value received. The codes are identified as a-research or institutional support; b-miscellaneous funding; c-royalties; d-stock options; e-consultant or employee; n-no conflicts disclosed, and *disclosure not available at time of printing.
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing.