
OTA 2011 Posters

BEST TRAUMA RELATED POSTER – 2011 ORS MEETING
RSA Evaluation of an Implant System for Above the Knee Amputee Patients
Charles Bragdon, PhD1; Anne Antonellis, BA1; Rickard Branemark, MD;
Johan Kärrholm, MD, PhD; Orjan Berlin, MD; Henrik Malchau, MD, PhD1;
1Harris Orthopaedic Laboratory, Massachusetts General Hospital,
Boston, Massachusetts, USA;
2Salgrenska University Hospital, Gotborg, Sweden
Introduction: Rehabilitation of patients with high, above the knee amputations poses a challenge due to the fact that the standard socket prosthetic devices are difficult or, in some cases, impossible to use. Over the past 15 years, the development of transdermal osseointregrated devices that allow for the application of external prosthetic devices has shown to be a promising solution for these difficult cases. A system has been developed in which a fixture is implanted into the distal femur of the amputee and allowed to osseointregrate into the bone. This fixture is connected to a skin penetrating abutment. An external prosthetic device is able to be easily connected to and disconnected from this abutment. The purpose of this clinical study is to assess the long-term fixation of these components in the femur using radiostereometric analysis (RSA). The RSA system utilizes two simultaneous stereo radiographs that are analyzed using the UmRSA Analysis software (RSA Biomedical, Umea, Sweden).
Figure 1. Patient with OPRA prosthetic system.
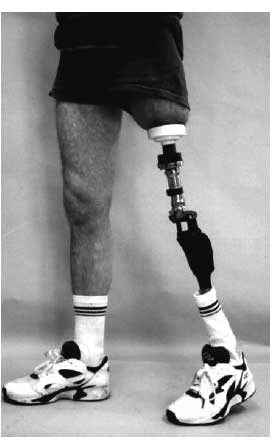
Methods: Fifty one patients, 28 males and 23 females, with high, above the knee amputations were enrolled into an RSA clinical study. Four of these patients had bilateral amputations, meaning 55 prosthetic systems were enrolled and followed in this study. The procedure for implanting the Osseointregrated Prosthesis for the Rehabilitation of Amputees, (OPRA), involves a two stage surgical procedure. The stage one surgeries took place between May 11, 1999 and December 11, 2007. The average age at time of surgery was 44.9 yrs (range 20.5 to 65.3), while the average Body Mass Index (BMI) was 24.7 (range 15.6 to 37.6). In the first stage the fixture piece of the prosthesis was implanted into the distal femur and the skin incision closed. Tantalum beads were placed in the bone and the implant during this first stage. After 6 months, the muscle and skin were trimmed, the abutment was connected to the femoral fixture, and the skin was closed around the distal end of this permanent portion of the device. A removable prosthetic device was then attached to the abutment and a rehabilitation program was initiated. Plain and RSA radiographic follow up was planned for 6 months, 1, 2, 5, 7 and 10 years after the second stage surgery. Plain radiographs were graded in 5 categories: distal bone resorption, near bone resorption, cortical thinning, cancellization, trabecular streaming or buttressing. Distal bone resorption is resorption of the distal bone causing exposure of the femoral fixture. Near bone resorption is resorption of bone around the fixture with a radiolucent zone being wider than the fixture thread depth. Cortical thinning is a decrease in the width of the cortex along the area of the bone where the fixture is implanted. Cancellization is an increase in the porosity of the cortex surrounding the fixture. Trabecular streaming or buttressing is defined as increase trabecular density at the proximal end of the implant, forming an angle between the inner cortex and the implant. The bone surrounding the implant is divided into Zones A-D and 1-12. Each follow up x-ray is graded in all 5 categories in the appropriate zones.
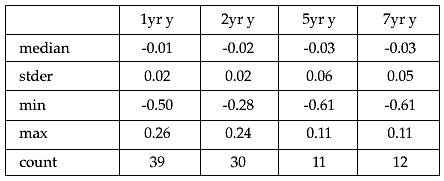
Results: As of February 2010, we have radiographic follow up and analysis on 40 patients at 6 months, 39 patients at 1 year, 30 patients at 2 years, 11 patients at 5 years, 12 patients at 7 years and 3 patients at 10 years. Nine patients’ films were unable to be analyzed in UmRSA due to inadequate visualization of the tantalum beads. One patient was deceased, one was excluded from the study after implantation due to issues with an implant on the nonsurgical side, three were explanted due to loosening, and one was excluded due to infection. Due to the low number of patients with 10 year follow up, the 10 year data is not reported at this time. Analysis from the UmRSA software showed that the median ± standard error of the proximal/distal migration of the device was -0.01±0.02 mm at 1 year; -0.02±0.02 mm at 2 years, -0.03±0.06 at 5 years, and -0.03±0.05 mm at 7 years. A Mann-Whitney test showed no significant difference in the median proximal/distal migrations at any follow up. The median ± standard error of the rotational movement was 0.04±0.17 degrees at 1 year; -0.05±0.17 degrees at 2 years; 0.42±0.38 degrees at 5 years; and 0.18±0.82 degrees at 7 years. A Mann-Whitney test showed no significant difference in the median rotations at any follow up time.
Table 1. The proximal/distal migration data of (mm) each patient. No implant had clinically significant progressive motion over the follow up period.
Plain radiographic analysis showed that cortical thinning occurred in 1 or more zones in 55% of patients by 5 years. Patients having cancellization in 1 or more zones decreased from 64% at 2 years to 55.5% at 5 years. At 2 years, 22% of patients showed trabecular streaming in 1 or more zones which increased to 50% by 5 years. Distal resorption was not a significant incidence as 0 patients showed any distal resorption by 5 years.
Discussion: The current RSA analysis of the OPRA system shows no significant migration or rotation of the implant in the bone, and therefore confirms a rigid fixation of the implant system. The surrounding bone remodeling did not compromise implant fixation or performance. The surgical technique for implanting the fixture and securing the skin to the abutment to avoid skin/implant motion and infection has been perfected over 10 years. The OPRA system is a promising new technique for addressing the prosthetic challenges faced by patients with high, above the knee amputations.
Alphabetical Disclosure Listing (628K PDF)
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant
.