OTA 2009 Posters
Scientific Poster #101 Tibia OTA-2009
Retropatellar Technique for Intramedullary Nailing of Proximal Tibia Fractures: A Cadaveric Assessment
Jonathan Eastman, MD (n); Susan Tseng, MD (n); Eddie Lo, MD (n); Chin-Shang Li, PhD (n); Brad Yoo, MD (n); Mark A. Lee, MD (4, 7-Synthes, Zimmer);
University of California, Davis Medical Center, Sacramento, California, USA
Purpose: A retropatellar technique combines advantages of the semi-extended technique with the percutaneous nature of traditional transpatellar nailing. The purpose was to investigate whether the anatomic start site or appropriate sagittal plane entrance vector is obtainable via a retropatellar technique.
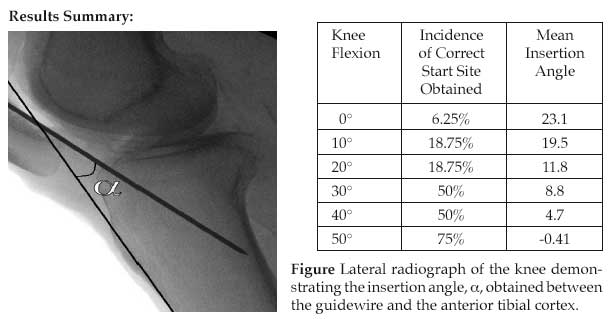
Methods: 16 paired cadaveric lower extremities were instrumented. Access into the suprapatellar pouch was performed through a small incision at the superior pole of the patella. A modified insertion trochar was positioned posterior to the patella and directed toward the superior tibial surface. Utilizing biplanar fluoroscopy, attempts were made to localize the proper anatomic start site, as defined by Tornetta et al. A Kirschner wire was inserted through the trochar. The wire’s angle with respect to the anterior tibial cortex was measured (see figure). The process was repeated at 0°, 10°, 20°, 30°, 40°, and 50° of knee flexion.
Results: The data are tabulated in the table below. The difference between the 0° and 50° subsets was significant (P = 0.00098). The difference in the mean insertion angles was statistically significantly different between each degree of knee flexion (P <0.0001)

Conclusion: The anatomic start site for tibial nails is attainable via a retropatellar approach with greater success at increasing degrees of knee flexion. As the knee is flexed, the entrance vector becomes more collinear with the anterior cortex. This parallelism could aid in minimizing sagittal plane deformities, especially during treatment of proximal shaft fractures.
Disclosure: (n=Respondent answered 'No' to all items indicating no conflicts; 1=Board member/owner/officer/committee appointments; 2=Medical/Orthopaedic Publications; 3=Royalties; 4=Speakers bureau/paid presentations; 5A=Paid consultant or employee; 5B=Unpaid consultant; 6=Research or institutional support from a publisher; 7=Research or institutional support from a company or supplier; 8=Stock or Stock Options; 9=Other financial/material support from a publisher; 10=Other financial/material support from a company or supplier).
• The FDA has not cleared this drug and/or medical device for the use described in this presentation (i.e., the drug or medical device is being discussed for an “off label” use). ◆FDA information not available at time of printing. Δ OTA Grant.